usp class vi vs iso 10993
This post will take a deeper look at what biocompatibility is and how it is defined by the International Standards Organization. You might establish biocompatibility via making the device of a Recognized Consensus Standard material using a validated process that does not degrade that material or by ISO 10993 testing.
ISO-10993 is a standard that utilizes systemic toxicity and intracutaneous reactivity testing.
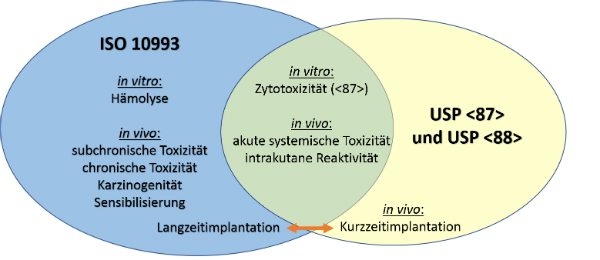
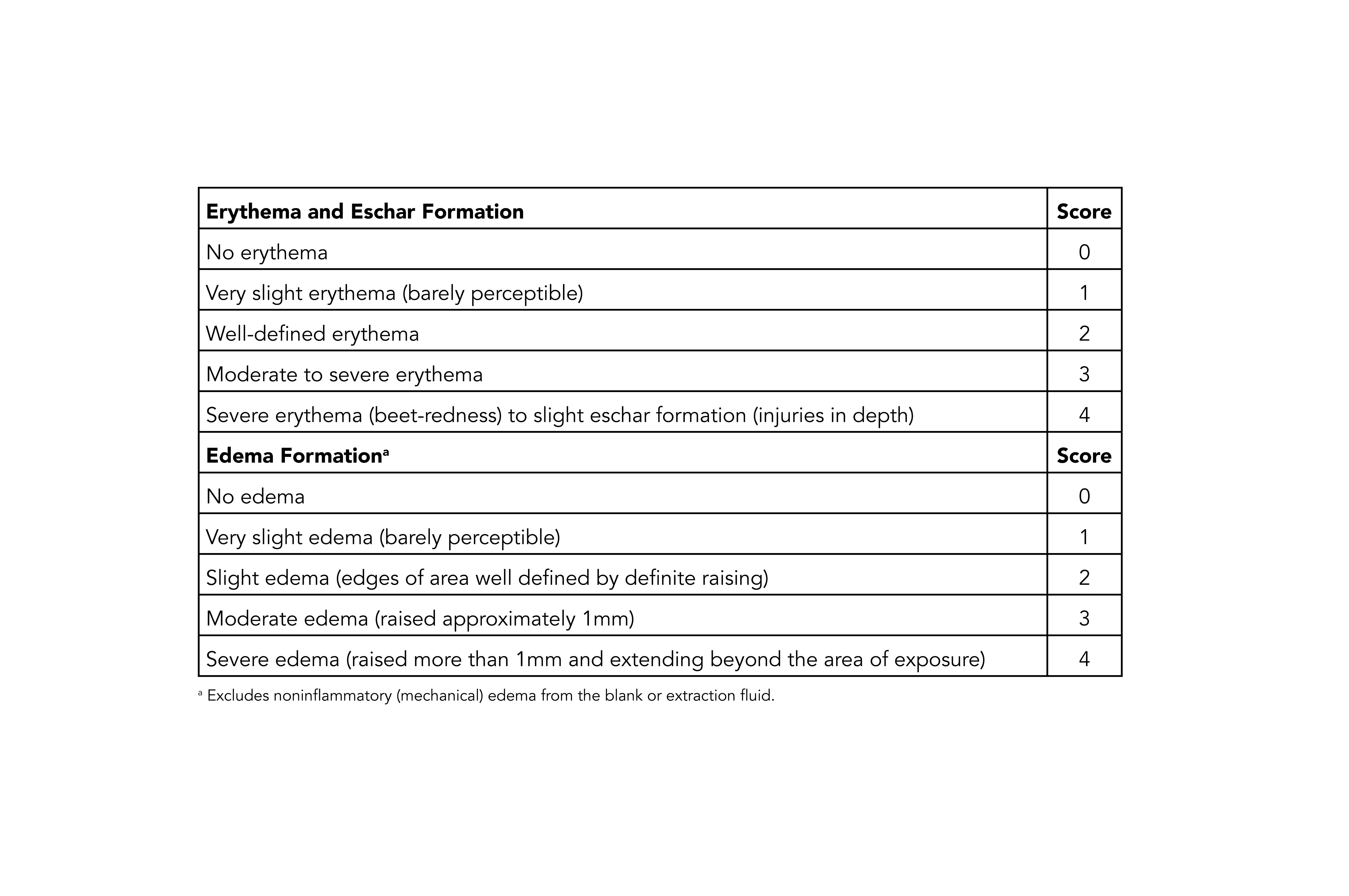
. While it is possible a USP Class VI material could also be ISO 10993 compliant its not a given and USP Class VI alone is not sufficient for adherence to ISO 10993. Duration similar to ISO-10993 and each class has a different set of testing requirements. USP Class VI demands an intracutaneous irritation test.
3D printing of one day crown prep guides. USP Class VI vs. A number of our plastic materials are ISO-10993 or USP Class VI capable.
That said the lack of risk assessment in USP Class VI can be a problem. For the purpose of the ISO 10993 family of standards biocompatibility is defined as the ability of a. USP Class VI Testing is only one standard of biocompatibility however.
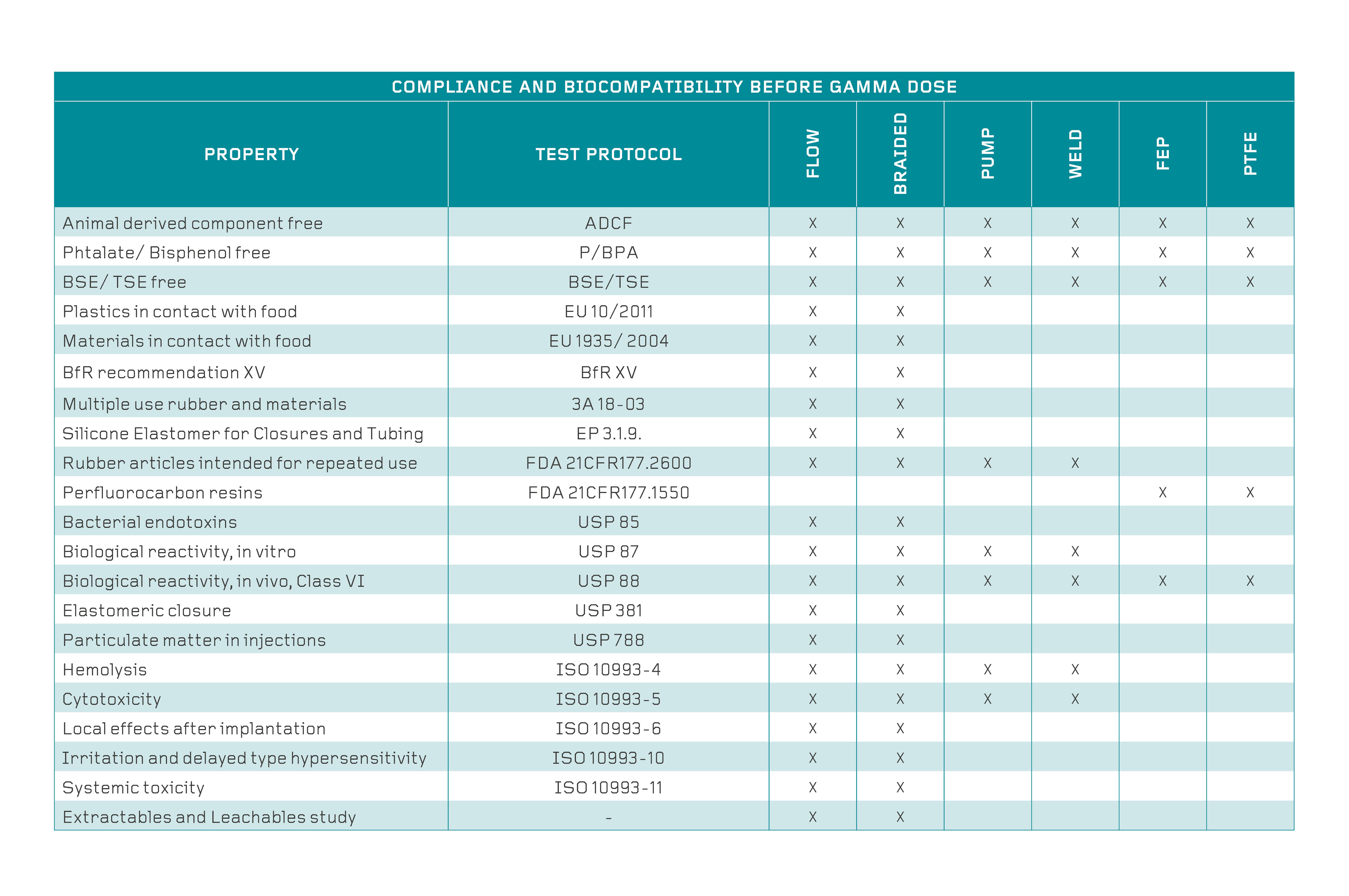
We carry a wide range of materials from the worlds top medical resin suppliers including USP Class VI and ISO 10993 certified biocompatible resins with full FDA Master File support. Evaluation and testing within a risk management process. USP Class Testing standards are determined by the United States.
In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts. Biological evaluation of medical devices Part 1.
Depending on the devices use the sterilization process might obligate you to do ISO 10993. Up-to-date materials manufacturers provide both USP and ISO 10993 test data to support both pharma and device customers. Many medical device companies are familiar with USP Class VI but that standard isnt as strict as ISO 10993.
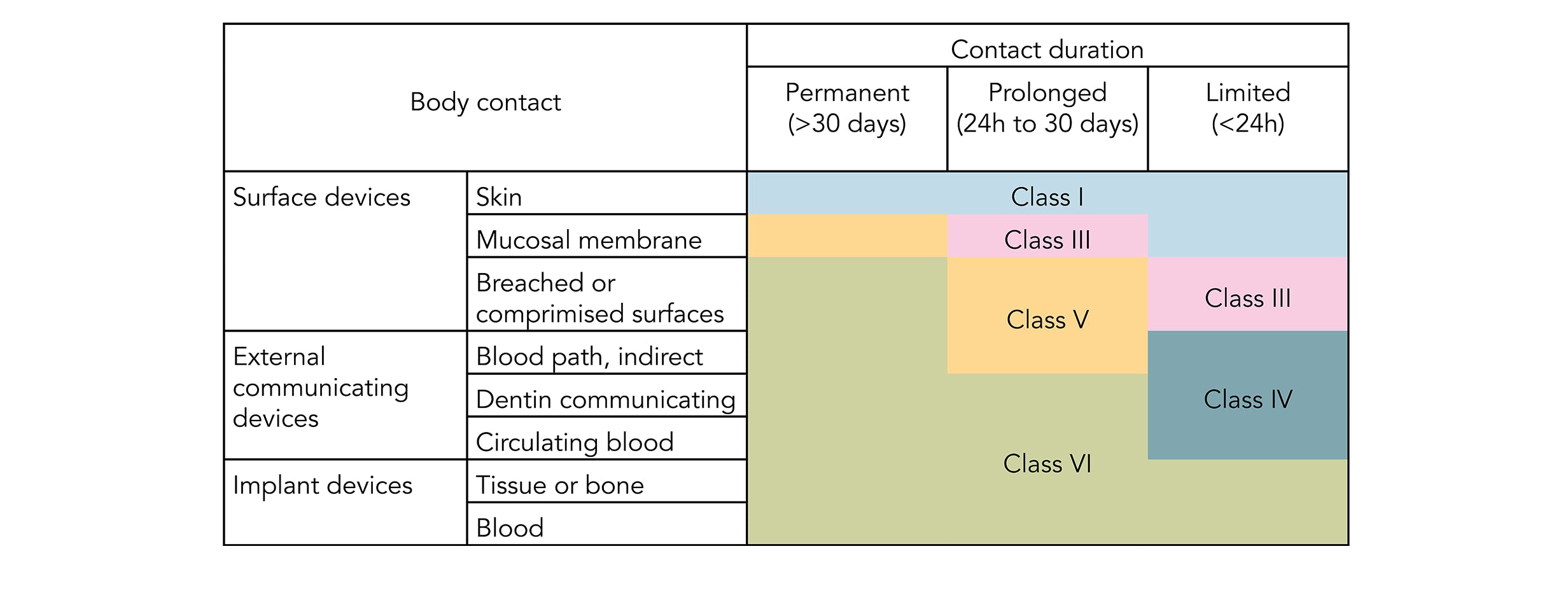
Our portfolio approach offers the most expansive selection of medical resin materials in the industry balancing performance cost. However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. In fact USP Class VI is sometimes seen as a minimum requirement for biocompatibility.
For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. 3D printing of dental and orthopedic surgical guides. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI.
These documents were preceded by the Tripartite agreement and is a part of the international harmonisation of the safe use evaluation of medical devices. Testing for medical devices should be conducted using rinsingeluting and sampling techniques as described in ISO 10993-1 14 and ISO 10993-12 15 as. USP class qualification no longer plays any role in medical device materials evaluation.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10 or USP Class VI testing. USP Class VI Testing is only one standard of biocompatibility however. The guidance memo wasis G95-1.
If yes to the first question then USP Class VI is not a relevant qualification for it. It depends to a large extent on the application and therefore also on the application period of the finished product. A more rigorous standard for the biological evaluation of medical devices is ISO-10993.
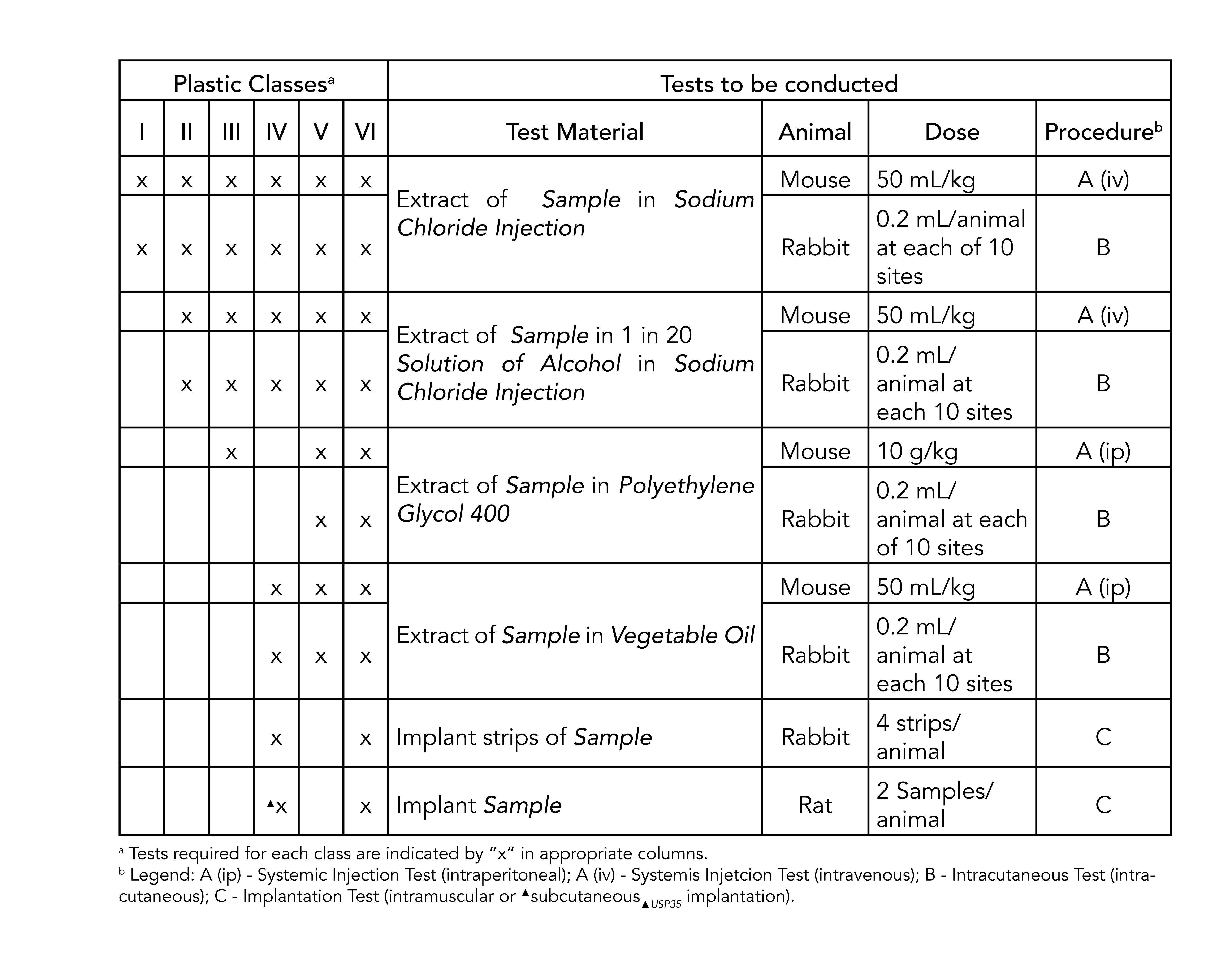
Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI. There are six classes VI being the most rigorous. That being said if you cant get an ISO 10993 compliant material often because the material simply hasnt been tested using a USP Class VI material is a less risky option.
In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. Most applications are fairly benign to elastomers. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials.
USP Class VI. The ISO 10993 set entails a series of standards for evaluating the biocompatibility of medical devices to manage biological risk. Unlike other rubber standards theres no one standard that engineers use for an approval.
ISO 10993 is designed for medical products that remain permanently or for a very long time in the human body so for shorter applications a USP Class VI or even a lower USP Class certification is often sufficient. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. USP Class VI Regiment Irritation Systemic Injection Implantation 1 week Biological Evaluation Plan BEP.
Class VI and ISO 10993 are recommendations for testing based on the use of the final device. Typically the terms USP Class VI or ISO 10993 materials are used. Though not a limited series of tests some biocompatibility requirements for medical devices may exceed the testing performed in USP Class VI.
A rubber compound has set physical parameters it needs to meet. The two most common test regimens commonly used to measure biocompatibility are USP USP Biological Reactivity Testing USP Class VI and ISO 10993 for the Biological Evaluation of Medical devices. To begin let us address just what biocompatibility is.
The materials listed below are ideal for. A more rigorous standard for the biological evaluation of medical devices is ISO-10993. A more rigorous standard for the biological evaluation of medical devices is ISO-10993.
Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by gamma irradiation and autoclave Product Validation Test Summaries available upon request Moldable bondable and formable for single-use assemblies and overmolds Temperature. Systemic injection test Intracutaneous test Implantation test USP standards for the first two tests in the list above are nearly identical to ISO-10993 standards for. For this reason the FDA provides a standard 21 CFR1772600 defining allowable rubber compound ingredients and extractibles based on toxicity and carcinogenicity.
This post will focus specifically on USP my next post will focus on USP. This is their current stance today. Take an ASTM D2000 call out.
So does ISO 10993. The most stringent Class VI requires three types of tests.
Biocompatibility Of Plastics Zeus
Petg Usp Class Vi Iso 10993 1 Medizinisches Biokompatibilitat Zer

Biokompatibilitat Ein Massstab Fur Usp Class Vi Reichelt Chemietechnik Magazin
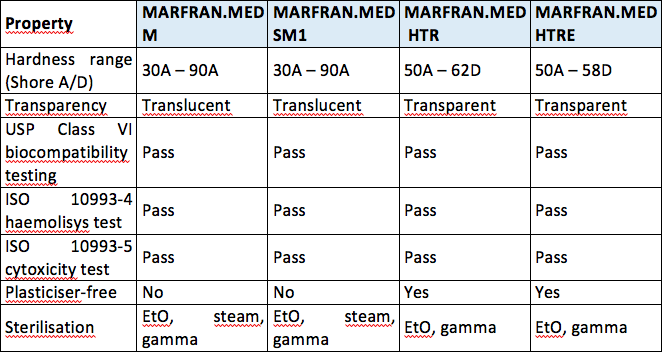
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Usp Class Vi Foster Corporation

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Zulassung Was Bedeutet Das Reichelt Chemietechnik Magazin

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group